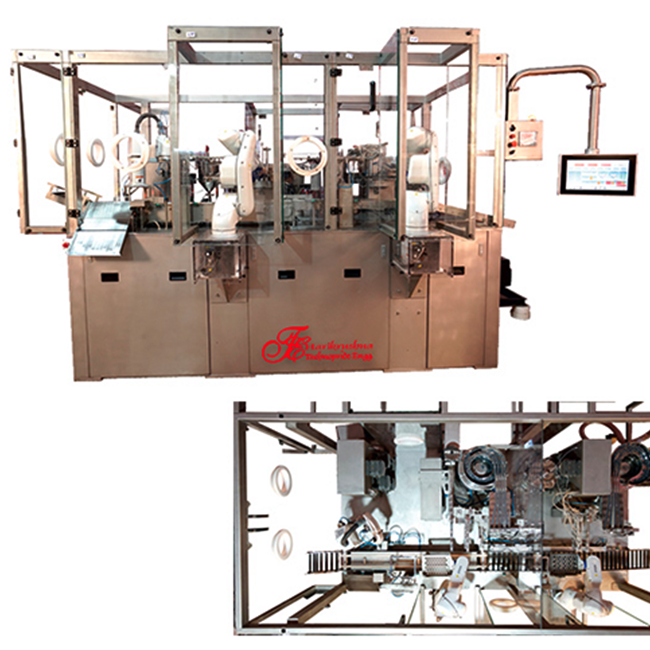
Dual Chamber Syringe Filling & Stoppering Machine Manufacturer
| MODEL | HTE-DCS-50 ISD |
| FILLING DOSE | LIQUID/POWDER |
| OUTPUT | 50 SYRINGES/MIN (DOUBLE CHAMBER SYRINGE) |
| OPTIONAL EQUIPMENT | AUTOMATIC LOADING / UNLOADING OF THE NEST. |
| VACUUM-ASSISTED LIQUID FILLING* | PREVENT AIR TRAPPED IN THE LUER CHANNEL. |
| VACUUM-ASSISTED PLUNGER INSERTION FOR | RIGID OR TEFLON COATED PLUNGERS |
| CHECK WEIGHING OF THE DOSE. | 100% IN PROCESS CHECK SYSTEM |
| GAS FLUSHING BEFORE, | DURING OR AFTER THE FILLING PROCESS. |
| DOSING SYSTEM FOR | CIP / SIP CONDITIONS. |
| SCADA SOFTWARE TO PROCESS DATA ACQUISITION | IN ACCORDANCE WITH FDA 21CFR PART 11. |
| MONITORING AND PARTICLE COUNTING. | |
| LAMINAR FLOW / RABS / ISOLATOR. IQ / OQ VALIDATION PACKAGE. | |
| CONTAINER TYPE | PFS / CARTRIDGE/ VIALS ALSO CAN BE FILLED IN THE SAME MACHINE*. |
Dual Chamber PFS Filling and Stoppering Machine
In the manufacturing of pharmaceuticals, a Dual Chamber PFS Filling and Stoppering Machine is an essential piece of machinery, particularly when producing prefilled syringes (PFS). These devices provide versatility in production operations by having the capacity to fill and stopper two distinct pharmaceutical solutions or drugs at the same time. To provide perfect dosage and sterility, a Dual Chamber PFS Filling and Stoppering Machine operates through precision automation. The apparatus has sophisticated pumps or dosing systems that accurately fill prefilled syringes with predetermined dosages of medication. To preserve the integrity of the product and avoid contamination, this process is carried out under carefully monitored circumstances. After the syringes are filled, the machine automatically seals them and incorporates stoppering mechanisms that guarantee the integrity and sterility of the finished product. Strict quality control procedures are followed throughout the process to monitor critical variables including sterility and volume correctness. Through an intuitive interface, operators can monitor the production process, adjust parameters and take appropriate action when necessary. Efficient cleaning and maintenance is facilitated by the design of the machine, which is essential to maintain GMP (Good Manufacturing Practice) standards and legal compliance. All things considered, a dual chamber PFS filling and stopping machine simplifies the manufacturing process of prefilled syringes by automating critical processes while maintaining strict criteria for precision, sterility and quality assurance. Pharmaceutical companies looking for reliable and efficient injectable drug manufacturing processes need this technology.
